 Diamonds are among the most valuable materials on the planet. Their strength, unparalleled by any other naturally occurring mineral, gives them practical uses in cutting and polishing tools.
Diamonds are among the most valuable materials on the planet. Their strength, unparalleled by any other naturally occurring mineral, gives them practical uses in cutting and polishing tools.
But of course, they are best known for their appearance; they’ve earned a reputation on high-end jewelry and engagement rings, in particular.
But now, they may provide us with a value of a different kind: scientific discovery. How can the discovery of new diamonds yield insights into the Earth’s water? Read more to find out.
How Diamonds Are Formed
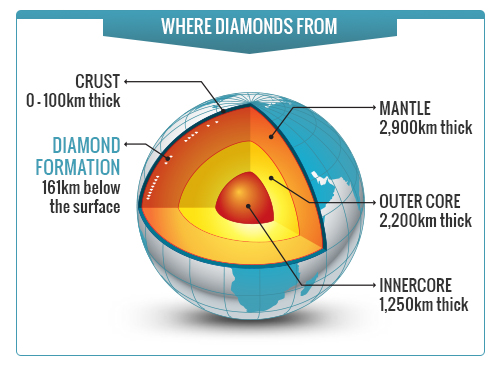 Diamonds are mainly formed around or below one hundred miles beneath the Earth’s crust, in the Earth’s upper mantle and transition zone.
Diamonds are mainly formed around or below one hundred miles beneath the Earth’s crust, in the Earth’s upper mantle and transition zone.
With all those tons of rock piled on top, substances containing the element carbon begin to shift and change under immense pressure. Deep enough into the Earth, the weight becomes so heavy that the very chemical structure of the carbon compound changes.
Favorable conditions allow for several carbon atoms to bond in the specific crystalline structure of a diamond. More and more carbon atoms attach to the lattice structure until billions of atoms have lined up in the correct configuration, and a sizable diamond has formed.
However, as carbon atoms are added to the ever-growing crystal, impurities find their way into the gaps and skew the structure slightly. One of these impurities is the liquid water found in the upper mantle and transition zone, which becomes ice when trapped in a diamond and put under pressure.
When the diamond emerges at the Earth’s surface, scientists who find it can study the ice to learn more about the composition of the upper mantle and transition zone.
Water Trapped
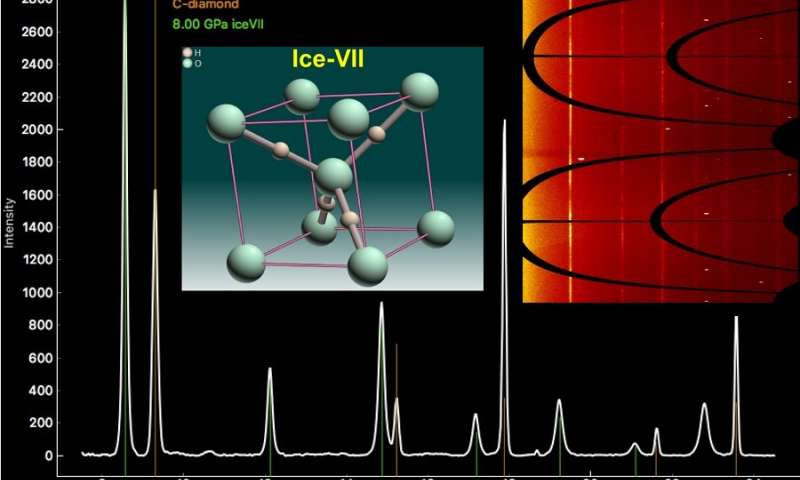 Recently, in the diamonds, scientists discovered a special type of ice, ice-VII, which forms under immense pressure combined with a drop in temperature. They use special x-ray techniques to identify the chemical composition of the various impurities in diamonds.
Recently, in the diamonds, scientists discovered a special type of ice, ice-VII, which forms under immense pressure combined with a drop in temperature. They use special x-ray techniques to identify the chemical composition of the various impurities in diamonds.
Researchers believe that the water got trapped in the diamonds during formation, and as the diamonds traveled up closer to the surface, the temperature dropped (thus turning the water to ice) while the pressure of the strong diamond structure remained.
Examining the diamond led researchers to believe that the diamond was formed in the transition zone, which implies that liquid water can be found in that layer of the Earth. The existence of water in the transition zone is not an entirely new discovery in geology, but this study certainly adds new information to the topic.
The study, published in the magazine Science, claims that this new information may soon “play a key role in the global water budget.” Extracting significant amounts of water from hundreds of miles under the Earth’s surface, though, requires still more innovation and discovery. For now, we’ll stick to diamonds!
Sources: Phys.org, Gizmodo, Smithsonianmag, Sciencedaily, Wikipedia







